Biology Forum › Cell Biology › ATP
- AuthorPosts
- November 18, 2005 at 12:21 am #2567
 kassia420Participant
kassia420ParticipantHow, specifically, does ATP store energy?
- November 18, 2005 at 12:57 am #33009
 EminemParticipant
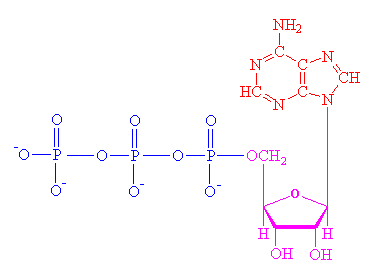
EminemParticipantATP refers to Adenosine triphosphate.
It contains ribose sugar, adenine base, and phosphate group, PO4-2
here is a diagram of its molecular structure

so basically, the phosphate group on the far left breaks off when an enzyme tell it to do so. This reaction releases energy. Its tat simple
After it breaks, adenosine diphosphate (ADP) and orthophosphate (HPO4) as the products.

The conversing of ATP to ADP is a cycle, where ADP with energy and that phosphate group can produce ATP.
Therefore, if the phosphate group dont break off, it can store the energy until a enzyme tells it to
- November 18, 2005 at 2:56 pm #33044
 thank.darwinParticipant
thank.darwinParticipantIt’s stored in the high energy bonds between the phosphates; P-P-P
- November 18, 2005 at 3:27 pm #33046
 Terry K.Participant
Terry K.ParticipantYes, there are high energy bonds stored between the phosphates, but the adenosine has something to do with the storage as well, but I don’t remember how, the internet should help you out more than I can. Just search for energy storage in ATP and you should get some helpful sites.
- November 19, 2005 at 12:10 pm #33090
 victorParticipant
victorParticipantATP + O2 ➡ ADP + Pi δH = -7.3 kkal/mole (in vitro)
ATP + O2 ➡ ADP + Pi δH = -13.3 kkal/mole (in vivo)Can anyone explain about this? 😉
- November 19, 2005 at 5:32 pm #33101
 MrMisteryParticipant
MrMisteryParticipantShouldn’t the in vitro energy be bigger than in vivo?
- November 24, 2005 at 1:08 am #33380
 burningredphoenixParticipant
burningredphoenixParticipantyou must understand that it doesn’t store energy, it merely releases energy as the bonds are broken
- November 26, 2005 at 10:51 am #33576
 quasi426Participantquote victor:ATP + O2 ➡ ADP + Pi δH = -7.3 kkal/mole (in vitro)
quasi426Participantquote victor:ATP + O2 ➡ ADP + Pi δH = -7.3 kkal/mole (in vitro)
ATP + O2 ➡ ADP + Pi δH = -13.3 kkal/mole (in vivo)Can anyone explain about this? 😉
The concentrations of product and reactant also play a role in the energy released.
- AuthorPosts
You must be logged in to reply to this topic.