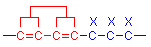Biology Forum › Molecular Biology › chromophores
- AuthorPosts
- February 15, 2007 at 4:54 am #6955
 sarahjay8Participant
sarahjay8ParticipantI’m not sure if I put this topic in the right place so if not, Sorry!
Does anybody know if the following qualify as chromophores? I can not find any information relating to individual atoms in a molecule. Some assistance would be muchly appreciated:
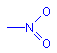
The nitrogen of amines? (n to σ* transitions)
the oxygen of alohols? (n to σ* transitions)
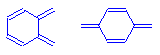
Aromatic compounds? ( π __> π*) <—thats a pie sign
All organic compounds (σ—->σ* transitions)thanks in advance!
- February 15, 2007 at 9:37 am #68953
 dipjyotiParticipant
dipjyotiParticipantChromophores are atomic configurations which can alter the energy in delocalised systems. They are composed of atoms joined in a sequence composed of alternating single and double bonds. Double bonds in organic compounds can be of two types. If the atoms with double bonds are not adjacent, they are termed isolated double bonds, and exist independently of other double bonds in the same molecule. If adjacent atoms have double bonds they are termed conjugated double bonds and the bonds interact with each other. Chromophore configurations often exist as multiple units, having conjugated double bonds, and are more effective when they do so. This is due to the interaction between the double bonds, which causes partial delocalisation of the electrons involved in the bonds. In this case, although specific atoms are involved in the bonds, the electrons are distributed over a larger area than the specific atoms and also involve adjacent atoms that have double bonds.
Thank You
Dip Jyoti Chakraborty
- February 15, 2007 at 9:48 am #68954
 dipjyotiParticipant
dipjyotiParticipantThe nitrogen of amines. (n to σ* transitions)
COMPLEFXO RMATIBOETNW EEN AMINESA ND HALOMETHANES
from 1 to 4 X loTa dynes and thus not significant. experiments made on the same sample indicates that little
cision in bound water determination varied from 1 to 5% modified form was produced by drying and rehydradepending
on the amount of water bound. tion.
Acknowledgments.-This work was supported by
the Office of Naval Research through contract NOTE ADDED IN PRooF.-Using the auto-oxidation and
carbon-monoxide-binding tests the OCy preparations were 710(15) NR 304-306 with the University Of
found to contain 95% native form. The oxidation-reduc- Minnesota and by the United States Public
Pre-
The field factor
, was 5.41 X 106 gauss’lcm.
tion titration behavib; was that to be expected for a oneelectron
process and the standard half-cell potential had
the accepted value. Although no tests for the presence of
“modified” form after the hydratioll experiments were
made, the complete reproducibility of the susceptibility
Health Service. We appreciate this assistance. we wish to express our gratitude to professors
Doyle Britton and 2.2. Hugus for helpful consultations.
[CONTRIBUTION FROM THE SHELL DEVELOPMFCCONMTP ANY, EMERYVILLCEA,L IFORNIA]
Solvent Effects on n -+ Q* Transitions; Complex Formation Between Amines and
Halomethanes
BY D. P. STEVENSOANND G. hl. COPPINCER
RECEIVEDJU NE 30, 1961
By means of measurements of the ultraviolet absorption spectra of isoactane solutions of triethylamine with added, (1)
chloroform, (2) fluorotrichloromethane, (3) carbon tetrachloride and (4) bromotrichloromethane, it is shown that amines (B)
form complexes with halomethanes, (A), that are either 1: 1 charge transfer complexes or interact (in 1: 1 pairs) to give
rise to so-called contact charge-transfer spectra. The complex formation (or contact interaction) accounts for the photochemical
instability of solutions of aliphatic amines in carbon tetrachloride solution. The photo reactivities of the amines
in the three tetrahalomethanes in the ultraviolet increase in the order; FCCL < CICClr < BrCCb.
In connection with our survey of the use of solvent
effects on the spectral location of n + o*
transitions as means of studying specific solvation
of the hydrogen bonding type, it seemed to be of
interest to compare by this technique the hydrogen
bonding power of chloroform toward amines with
that of the previously studied water’ and simple
alcohol1b solvent systems. To this end we undertook
the comparison of the ultraviolet absorption
spectrum of triethylamine in chloroform solution
with that of this base in water, isooctane and diethyl
ether. In line with out previous experience, we
expected to find a blue shift of the chloroform solution
spectrum of the ethylamine relative to that of
the ether or isooctane spectrum only slightly less
than the blue shift that is found in tertiary butyl
alcohol solution.lb As may be seen in Fig. 1,
curves VI and V, respectively, the chloroform
solution spectrum shows a large red shift relative
to the isooctane solution spectrum, in complete
contradiction to our expectation.
This observation immediately suggested the
existence of an interaction between the amine and
chloroform that is quite independent of any hydrogen
bond complexing that these molecules may
undergo. As may be seen in curves VI1 and VIII,
the spectra of solutions of triethylamine in fluorotrichloromethane
and in carbon tetrachloride show
even larger red shifts from the isooctane solution
spectrum than does that of the chloroform solution.
Interaction here cannot involve hydrogen
bonding by solvent. This spectral behavior of
triethylamine in the halomethane solutions is that
which would be expected if the amine forms a complex
with the halomethanes of the type that have
become known as “charge transfer complexes.”2~a.4
(1) (a) D. P. Stevenson, G. M. Coppinger and J. W. Forbes, J. Am.
Chcm. Soc.. 88, 4350 (1961); (h) D. P. Stevenson, ibid., to be submitted
for publication.
In the following paragraphs we will present spectroscopic
evidence that triethylamine (B) does form
a one to one molecular complex with each of the
halomethanes (A) , chloroform, fluorotrichloromethane,
carbon tetrachloride and bromotrichloromethane,
of the type AB. We will also cite photochemical
evidence that shows such, AB, complex
formation is not limited to B = trialkylamine
and that the electronic absorption spectra of these
complexes are indeed very probably of the charge
transfer type.
The method employed to establish the existence
of triethylamine-halomethane complexes and to
determine the stoichiometry of the complexes was
the observation of the absorption spectra of dilute
isooctane solutions containing different ratios of
amine to halomethane. In concentration units
of moles/liter, the ratio B/A of the solutions
measured were approximately 0.75/0.25; 0.50/0.50;
0.25/0.75 and 0.25/0.25. Through the use of
calibrated quartz inserts in the 1 cm. cells it was
(2) H. A. Benesi and I. H. Hildebrand, ibid.. 71. 2703 (1949).
(3) R. S. Mulliken, J . Chem. Phys., 19, 514 (1951).
(4) The reviewer of this paper has suggested that the data to be
presented below are better interpreted as indicating that amines and
the halomethanes undergo “contact interaction ” of the type described
by L. E. Orgel and R. S. Mulliken [ J .A m. Chcm. SOC.7, 9, 4839
(1957)l to give rise to the new absorption hands as contact chargetransfer
spectra, rather than as actual charge-transfer complexes with
finite formation constants. The authors believe their erperimental
data are adequate to establish the stoichiometry of the interaction, 1: 1,
he it ordinary charge-transfer complex formntion or n case of contact
interaction. However, they also feel that it requires over interpretation
of the present data to reach a conclusion with respect to the question.
is the formation constant of the“comp1ex” very small, but finite,
or identically zero? The authors feel that the important aspect of the
present paper is their evidence that the halomethanes constitute a
hitherto unrecognized class of “acceptors” (albeit weak) for strong
donors of the amine type. It should be noted in this connection that
interaction of the type reported here is either non-existent or hardly
observable in the case of the diethyl ether-carbon tetrachloride system
(unpublished observations of one of the present nuthorn (DPS)). - February 15, 2007 at 9:56 am #68957
 dipjyotiParticipant
dipjyotiParticipantthe oxygen of alohols? (n to σ* transitions)???
I don’t get it. Is it alcohol?Thank You.
Dip Jyoti Chakraborty
- AuthorPosts
You must be logged in to reply to this topic.
No related posts.